Zhongya Hu , Simon V. Hohl , Sebastian Viehmann , Patrick Meister , Nathalie Tepe
a State Key Laboratory of Marine Geology, School of Ocean and Earth Sciences, Tongji University, Shanghai 200092, China
bInstitute of Mineralogy, Leibniz University Hannover, Callinstraße 1, 30167 Hannover, Germany
cDepartment of Geology, University of Vienna, Josef-Holaubek-Platz 2, 1090 Vienna, Austria
dCenter for Microbiology and Environmental Systems Science, Department of Environmental Geosciences, University of Vienna, Josef-Holaubek-Platz 2, 1090 Vienna, Austria
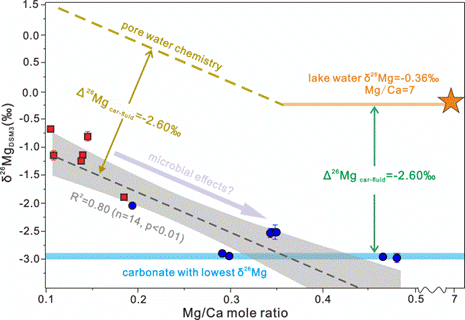
Abstract:The growth and morphology of stromatolites have been previously linked to diverse microbial activities. However, the role of microbial metabolisms on carbonate formation during stromatolite growth remains controversial. Magnesium isotopes have been proposed to serve as a tracer of microbial carbonate formation, implying a potential biological isotope fractionation. To further elucidate whether Mg isotope fractionation is modified during microbial carbonate formation, this study reports Mg isotope compositions of Holocene stromatolites and pore waters from Lagoa Salgada, a coastal ephemeral lake in Brazil. The stromatolitic carbonates are composed of high-Mg calcites characterized by extremely positive inorganic δ13C values, up to +20‰, and variable δ26Mg values, ranging from −2.98‰ to −0.68‰. Multiple pieces of evidence consistently demonstrate changes in microbial metabolism resulting from ecosystem fluid chemistry evolution. However, the direction of Mg isotope fractionation associated with carbonate precipitation remained invariable despite the changes in microbial activity. Mineralogical features indicate that the stromatolitic carbonates formed via 2-D nucleation. An isotopic mass balance calculation based on the observed variations in δ26Mg values and Mg concentrations of associated pore waters argues for an Mg isotopic equilibrium fractionation factor of −2.60‰ associating with the carbonate formation at Lagoa Salgada, well-matching experimental values for abiotic calcite precipitation. Thus, the observed δ26Mg variability of stromatolitic carbonates is primarily controlled by the changes of physicochemical conditions in the ambient fluid. We infer that during the formation of stromatolites, a consortium of different microbial communities produces extracellular polymeric substances, which serve as a substrate for carbonate nucleation from ambient fluid without significantly affecting the fractionation of Mg-isotopes. Our findings shed light on the effects of microbial processes on carbonate formation during stromatolite growth and improve the current understanding of the Mg isotope record in microbial carbonates.
Full Article: https://doi.org/10.1016/j.gca.2023.07.022