Chengfan Yang a*, Shouye Yang a, Nathalie Vigier b
a State Key Laboratory of Marine Geology, Tongji University, 1239 Siping Road, Shanghai 200092, China
b Oceanography Laboratory of Villefranche (LOV), CNRS, Sorbonne University, 06230 Villefranche-sur-Mer, France
Abstract:
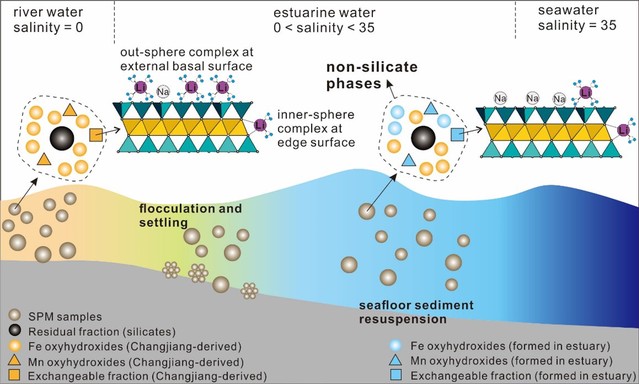
Lithium (Li) isotopes are widely used as a tracer of silicate weathering. However, Li isotopic compositions of non-silicate phases carried by sediments/suspended particulate matters (SPM) have not been systematically investigated, and their influences on the dissolved δ7Li variations remain poorly known. This study investigated Li geochemistry of different chemical fractions extracted by sequential leaching from SPM samples in the Changjiang (Yangtze River) Estuary. Our results demonstrate that Fe-Mn oxyhydroxide is the largest reservoir of non-silicate Li, accounting for ~5% of Li in bulk SPM samples. The proportion of Li in exchangeable fraction is only 0.3 ± 0.1%, with δ7Li values ranging from 14.2‰ to 22.4‰. In response to seawater addition, the dissolved Na progressively replaces physically-adsorbed Li, and the chemically-adsorbed Li is isotopically fractionated with a factor of 0.9883. In the Changjiang Estuary, δ7Li of Fe-Mn oxyhydroxides responds rapidly to environmental variations. The isotope fractionation factors during Li uptake into authigenic Fe- and Mn-oxyhydroxides are 0.981 and 0.9735, respectively. Possibly due to short interaction time during SPM passing through the estuary, incorporation of Li into non-silicate phases cannot drive a detectable alteration of estuarine dissolved Li concentration. By contrast, this study highlights the possibility of oxyhydroxides affecting δ7Li values of river water. Further work may focus on determining Li isotopic behaviours of non-silicate phases and their applications on environment reconstruction.
Full article:https://www.geochemicalperspectivesletters.org/article2133/