Di Cai1*, Hebin Shao2, Long-Fei Gou3,4, Zhangdong Jin4,3 Shouye Yang1
1. State Key Laboratory of Marine Geology, Tongji University, 1239 Siping Road, Shanghai 200092, PR China;
2. Key Laboratory for Polar Science of the State Oceanic Administration, Polar Research Institute of China, Shanghai 200136, China;
3. Department of Geography, Chang'an University, Xi'an 710054, China
4. State Key Laboratory of Loess and Quaternary Geology, Institute of Earth Environment, Chinese Academy of Sciences, Xi'an 710061, China
*Corresponding to: dcai@tongji.edu.cn
Abstract:
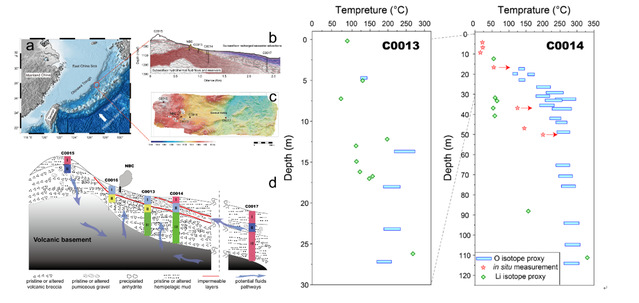
River water and submarine hydrothermal fluids are the two primary sources of lithium (Li) input to the ocean. However, compared to well-documented weathering processes, the behavior of Li isotopes during hydrothermal alteration processes remains obscure. In this research, we investigated the Li isotopic compositions (δ7Li) of hydrothermally altered sediments from two IODP drilling cores (C0013 and C0014) in the Iheya North Knoll of the middle Okinawa Trough. The sediments, primarily altered from volcanic clasts, exhibit a pristine δ7Li value (5.2 ± 0.7 ‰) similar to that of MORB (3.7 ± 1 ‰). Intense hydrothermal activity led to the complete conversion of primary feldspars into phyllosilicates, predominantly Mg-chlorite, at site C0013 and in the middle and deep parts of C0014. The altered samples showed a depletion of 7Li (ranging in δ7Li from -10.0 ‰ to 4.2 ‰) compared to background sediment, indicating the preferential incorporation of 6Li during the formation of secondary minerals. Furthermore, both drilling sites exhibited increasing trends in δ7Li values with depth, with the deepest samples approaching the pristine δ7Li values of unaltered volcanic rock. These trends were attributed to the significant thermal gradients present at both sites. The temperature-dependent fractionation factor of Li isotopes allowed us to estimate the alteration temperature, which yielded temperature values consistent with those obtained using the δ18O geothermometer. This finding underscores the potential of Li isotopes as a novel geothermometer tool, although further calibration work is required. Based on our research findings, we developed a dissolution-precipitation model (based on mass balance) that establishes a relationship between the δ7Li values of altered solids and hydrothermal fluids, with the aim of elucidating the relatively limited variation observed in global submarine hydrothermal fluids (ranging from 1.6‰ to 11.0‰, with an averaged value of 5.9 ± 3.0 ‰, mean ± SD). This model quantitatively depicted the strong restriction of the Li concentration in hydrothermal fluids on their isotopic compositions, as high concentrations of Li in the fluids necessitate a substantial release of Li from the solid phase, causing the fluids' δ7Li values to approach that of the bedrock. Additionally, limited fractionation factors at high temperatures further constrain the variations in the fluids' δ7Li values. Model predictions also suggest that the reported δ7Li values of hydrothermal fluids have encompassed the potential δ7Li variation, providing supporting evidence for the assumption that δ7Li values of submarine hydrothermal fluids have exhibited insignificant variations throughout Earth's history.
Full Article:https://www.sciencedirect.com/science/article/pii/S0016703724001182